Colloid-A-Tron:
A non-chemical water treatment system
by Dr D.M.Dawson, Ph.D, Harwell Laboratories’ Materials Development Division
The Colloid-A-Tron is a non-chemical water treatment system. It is operating effectively in preventing hard scale build-up in several thousand heat exchange plants around the world; a market that has been built-up over the last 40 years. This article describes the reasons for scale formation from hard waters and examines some recent research into the way the Colloid-A-Tron modifies the scaling behaviour of calcium carbonate. This research has provided clear, scientific evidence that the system has a significant effect on scale formation conditions in hard waters. The combination of field experience and basic scientific research programs is generating more confidence in preventing scale ‘physically” rather than using chemical water-treatment systems.
Scaling and its causes
Water is a very good solvent for minerals and many other materials. Natural waters are essentially “ionic soups”. All of the ionic species are trying to keep in a thermodynamic equilibrium with their environment, and they achieve this by combining together in clusters – perhaps growing to form crystals – or by breaking up into free ions. All of these reactions are occurring all the time as local conditions change.
Forming part of this “soup” are the ions calcium and carbonate, and they can form calcium carbonate – the principle scaling salt found in hard waters. We need to understand the equilibrium of these species in water, and in doing so we will know whether scaling can occur, or not. If we can, in some way, change the equilibrium with Colloid-A-Tron, we can modify the scaling behaviour of hard water.
The equilibrium solubility product for calcium carbonate is a thermodynamically defined value for a given pressure and temperature. It is the concentrations of calcium and carbonate free ions in equilibrium with a large crystal of calcium carbonate suspended in water. Equation (1) is the standard way of showing this equilibrium.
Equation 1:
[Ca ++ [CO3] -> [CaCO3]
It is this equilibrium point that all waters will try and reach either by crystal growth or dissolution. At this equilibrium point, the crystal will be growing and dissolving at the same rate.
Most waters will be at equilibrium if given time, but changes in the environment, such as bore-hole water emerging to the surface or the temperature and pressure changes that take place in heat-exchange plants will disturb this equilibrium. This leads to changes of the water composition. If the concentration of calcium and carbonate is greater than the equilibrium requirements, then the water attempts to reduce these concentrations and it does this by precipitation and growth of scale crystals. Equally, the water may be undersaturated with respect to calcium carbonate, and this will increase the free ion concentrations by dissolving scale crystals.
The term supersaturation ratio (Sr) is used as a shorthand description of the equation that determines whether the water can scale:
Sr = [Ca++]a [CO3–]a
[Ca++]eqm [CO3–]eqm
The square brackets refer to the ion concentrations (mole/litre) of the free calcium and free carbonate, the ‘a’ refers to actual concentrations prevalent in the water, and ‘eqm’ to the equilibrium concentrations as defined above.
If Sr > 1, scaling can occur; if Sr < 1, scaling cannot occur but dissolution can.
This is a fundamental, thermodynamic requirement for scale formation or dissolution. From it we can see that scaling is controlled by the equilibrium concentrations of Ca++ and CO3 ions, defined by pressure and temperature and by the actual free ion concentrations of these in any water.
All of these control factors can be modified to some degree for a given water and heat exchange plant. The one that is most readily, and economically, variable is the CO3- – ion concentration. Fig.la illustrates the overall carbonate equilibrium reaction path available in a hard water. Each reaction and ionic species demands its own equilibrium concentration. If the temperature is increased, the bicarbonate ion thermally decomposes forming carbonate, thereby increasing the supersaturation ratio and providing the conditions for scale formation. It is this reaction that causes the scaling of hot surfaces – whether the surface is a kettle element or a large heat exchanger. If the pH level increases, the water tries to produce free H+ ions so as to achieve the H+/OH equilibrium for H20 . One of the easiest ways it can do this is to decompose carbonic acid, and bicarbonate the ion – again producing carbonate ions and increasing the supersaturation ratio.
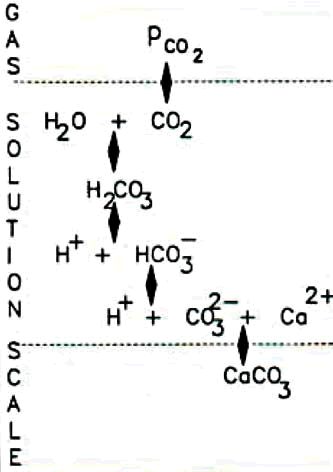
Fig 1a. The alkalinity equilibrium chain
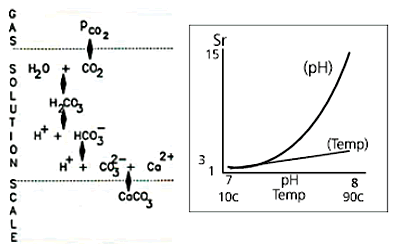 Fig
1b. Increased temperature and pH effects
Fig
1b. Increased temperature and pH effects
Increasing pH has a much greater effect on Sr than an increase in temperature, as illustrated in Fig 1b.
A temperature change of 80 degrees C takes a water that is close to saturated at 10 degrees C to a supersaturation ratio of between 3 and 4. This will be enough to cause severe scaling. But a change of one pH unit, from 7 to 8 increases Sr to around 15. Scaling is around 5 times as rapid – or five times as likely to occur – than under the temperature increase shown above.
pH levels and temperature are the factors that most affect the CO3 concentration, and in turn affect Sr and consequently scale formation; pH is the most dominant.
Can we then use these factors to propose, and demonstrate, a feasible explanation for the Colloid-A-Tron water treatment? The answer is yes.
Water Treatment
The Colloid-A-Tron unit ( Figs 2 and 3 ), never more than 1m long, is plumbed into the system it is meant to protect. The standard unit is an apparently simple device; water is made to flow past a special alloy insert in a length of pipe.
If the unit is to work, it must change the water as it flows past. It must, in some way, reduce the supersaturation ratio of the water, i.e. the free Ca++ and free CO3– ions must be reduced. The most obvious way would be by precipitating them as calcium carbonate – much like lime softening techniques.
Because of the composition of the unit, there are only two ways in which it can work. First, the special alloy could either adsorb ions from the solution or corrode. Either event could increase the pH locally, increase the Sr locally, and cause precipitation of calcium carbonate, thus providing the desired result that downstream of the Colloid-A-Tron the water is part-softened and carries suspended calcium carbonate crystals. Secondly, the shape of the unit promotes turbulence; the associated pressure differences could cause dissolution of CO2 gas which may get stripped from the water when it becomes open to the atmosphere, in a cooling pump for example, and therefore reduce the total carbonic species in the water.
The second method is independent of alloy composition, but copper or zinc inserts, for example, do not work in practice. We are therefore drawn to the first, the special alloy, effect mechanism.
A research program at the UKAEA Harwell laboratory was commissioned to study the behavior of a number of synthetic hard waters, simulating typical waters experienced in practice, under carefully controlled conditions. Waters 1 and 2 were hard waters ( 350ppm as CaCO3 ) at different pH levels, 3 was a less hard water ( 250ppm as CaCO3 ) and 4 was simulated sea water.
1) Does Colloid-A-Tron alloy give rise to increases in pH of the water?
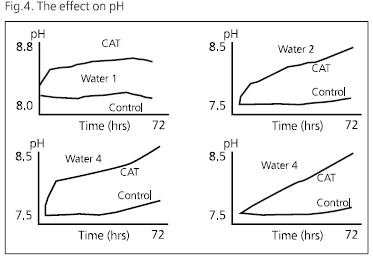
Colloid-A-Tron alloy granules were washed in distilled water, and were air dried and 35g samples were added to 500ml of synthetic water. The flasks were stoppered, shaken from time to time and the pH monitored. Fig 4 shows the results for each of the waters.
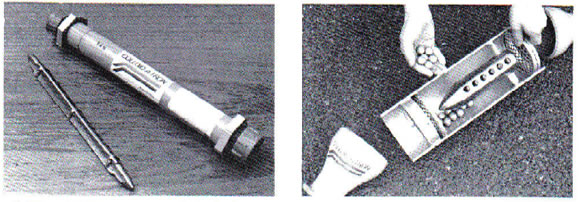
Fig.2 Fig.3.
Fig.2. The standard Colloid-A-Tron unit has a static triangular alloy core.
Fig.3. The 2000 unit showing the alloy spheres used to keep the core clean
Clearly, the alloy produces a significant increase in the bulk pH of the waters. Its effect on the water close to the unit’s surface may well be even more marked. Is this a sufficient change to produce significant bulk crystallisation of calcium carbonate and reduce the Sr of the treated water?
2) Does Colloid-A-Tron alloy cause precipitation in hard waters?
To answer this, Harwell conducted a series of simple experiments in which a metal spinner or impeller was rotated in hard water made metastable by injecting CO2 gas (Fig.5 illustrates the arrangement). The flask was open to air and as the CO2 was lost, the pH increased, until eventually crystal precipitation occurred and the pH decreased with the associated CO2 formation – this is very similar to threshold tests carried out by chemical water treatment companies.
 The
same four synthetic waters were tested and spinners
of copper (considered to be a material that was
inert – e.g., a control), zinc (to represent a
material that would be corroding fairly rapidly),
and Colloid-A-Tron alloy were used. The pH was
monitored continuously and a typical example of the
change in pH over time is also shown in Fig.5.
The
same four synthetic waters were tested and spinners
of copper (considered to be a material that was
inert – e.g., a control), zinc (to represent a
material that would be corroding fairly rapidly),
and Colloid-A-Tron alloy were used. The pH was
monitored continuously and a typical example of the
change in pH over time is also shown in Fig.5.
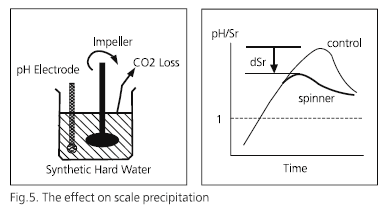 The
value of dSr – the difference between the Sr at
which the control system precipitates and that at
which the test impeller system precipitates – is a
measure of the effectiveness of the spinner at
promoting the production of scale crystals. The
larger this value, the greater the effectiveness of
the test alloy.
The
value of dSr – the difference between the Sr at
which the control system precipitates and that at
which the test impeller system precipitates – is a
measure of the effectiveness of the spinner at
promoting the production of scale crystals. The
larger this value, the greater the effectiveness of
the test alloy.
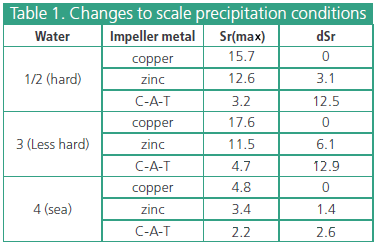
The results of these tests, as shown in Table I, are the definitive proof that the special alloy causes a significant change in scale crystal precipitation from hard waters. In hard waters (I and 2). less hard waters (3) and in sea water (4), Colloid-A-Tron has caused precipitation to occur at Sr values well below both the control values and those observed for a rapidly-corroding metal.
 The
combination of the results of these carefully-
controlled experiments, and recent interpretation –
in the light of the research results – of several
years of successes and failures in real industrial
applications, allows the following mechanism of
action to be proposed:
The
combination of the results of these carefully-
controlled experiments, and recent interpretation –
in the light of the research results – of several
years of successes and failures in real industrial
applications, allows the following mechanism of
action to be proposed:
The special alloy – by either adsorption and/or corrosion processes – produces an increase in pH in the water close to its surface, which is sufficient to cause CaCO3 crystal precipitation. The turbulence in the device minimizes the scale build up on the surface, and any CO2 dissolution will assist in the stabilization of the crystals. Hard water is therefore part-softened, and carries the crystals around the circuit harmlessly.
It must be stressed that the unit has not changed the total Ca and CO3 content of the water – it has merely tied up a proportion of the free ions as stable CaCO3 crystals which get carried round the circuit. These crystals will act as scale growth sites in preference to heat exchange surfaces, where conditions favor crystal growth, and so they may have positive benefits to keep surfaces scale-free. The suspended solids will settle out in low flow rate areas such as cooling tower sumps, and they can be removed during blow-down as required.
Summary
Scale formation is controlled by the thermodynamic equilibrium of the reaction:
[Ca++] + [CO3 — ]-> [CaCO3]
Colloid-A-Tron exploits this equilibrium by manipulating the local CO3 concentration and, thus, causes scale formation in the water near to the unit’s surface. The resulting crystals get carried harmlessly round the treated circuit.
Water treated in this way can be considered as part-softened and the heat-exchanger surfaces are, as a consequence, less at risk of scaling. This effect is becoming increasingly predictable through a combination of field experience and basic research programmes. The effect has now been built into the Fluid Dynamics water models which are incorporated in a specialist computerised prediction system which has resulted in significant improvements in the selection of appropriate installations and running conditions.
David Dawson studied materials science at Bath University.
After graduation in 1978 he joined the UKAEA (UK Atomic Energy Authority) Harwell Laboratories’ Materials Development Division, and he obtained his PhD in 1984 for his studies of the chemical inhibition of calcium carbonate scale nucleation and growth. In 1987 he joined industry to work on the development of ceramic materials.
He is currently also a consultant for Fluid Dynamics Ltd.

